INTRODUCTION:
One of the key actions in freeze drying is triple point. People are a bit confused with how this happens and why, and there is also a misconception that this is sublimation. The fact is that triple point is a stage you must achieve for the sublimation phase to start. Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase.
Triple Point Explained:
In physics and chemistry, the triple point of a substance is the temperature and pressure at which three phases (gas, liquid, and solid) of that substance may coexist in thermodynamic equilibrium.
Triple point of water:
Scientific explanation: The single combination of pressure and temperature at which pure water, pure ice, and pure water vapour can coexist in a stable equilibrium occurs at exactly 273.16 kelvins (0.01 °C) and a pressure of 611.73 pascals (ca. 6.1173 millibars, 0.0060373057 atm).
Simply put, the triple point of water is the only temperature at which water can exist in all three states of matter; solid (ice), liquid (water), and gas (water vapour). This temperature is 0.01°C.
At that point, it is possible to change all of the substance to ice, water, or vapour by making infinitesimally small changes in pressure and temperature.
To help explain this futher, here is a video which shows how one small change affects the equilibrium. (soruce: Science Channel)
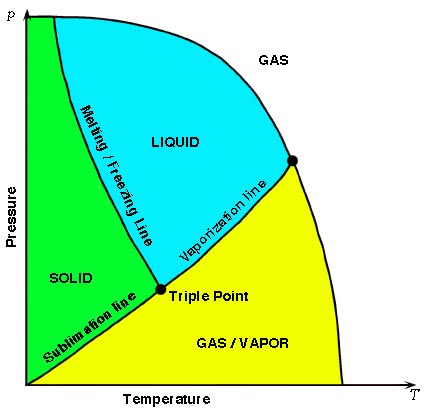
The image shows at what stage the triple point happens and how it will react when the equilibrium is changed.
For information on freeze drying, have a look at our web site – www.cuddonfreezedry.com
You can also download our free "Guide to choosing a Freeze Dryer"

